Launch of a New Kit for the Detection of Anti-Integrin αvβ6 Antibodies for Understanding Pathology of Inflammatory Bowel Disease (IBD)
Tokyo, Japan - April 4, 2022 - Medical & Biological Laboratories Co., Ltd. (President: Kimimasa Yamada), a JSR Life Sciences Company, will release a new kit for measuring anti-integrin αvβ6 antibodies for research use. The product was successfully developed in collaboration with Dr. Shiokawa, M., Kyoto University Graduate School of Medicine, under the support of the Japan Agency for Medical Research and Development (AMED). It is scheduled to be released worldwide on April 4th, 2022.
The number of patients with ulcerative colitis (UC) has been growing not only in Japan but worldwide. UC is a chronic inflammatory bowel disease and the underlying mechanism remains unclear. There are no specific biomarkers, and its diagnosis is determined comprehensively from clinical symptoms and tests such as endoscopy.
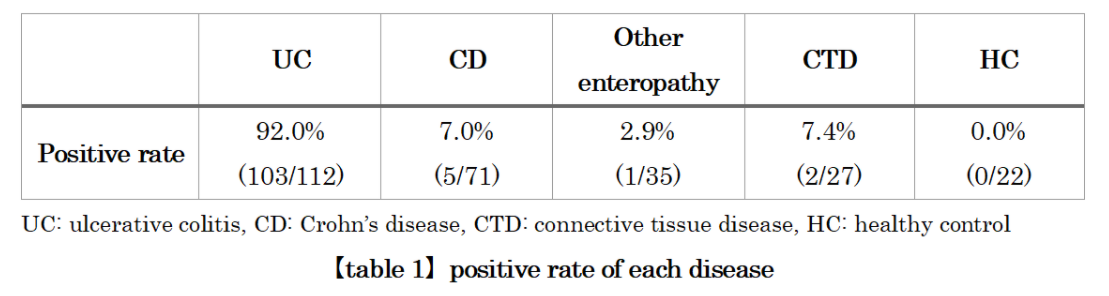
Anti-integrin αvβ6 antibodies are detected in about 90% of UC patients, and the results of basic research suggest that these autoantibodies themselves may be causing the disease.
The anti-integrin αvβ6 antibody is an autoantibody against integrin αvβ6. Integrin is a cell-surface receptor that is responsible for cell adhesion by binding to extracellular matrix. In mammals, there are 24 different types of integrin. Integrin αvβ6 expressed in the intestinal epithelium is associated with suppression of inflammation and prevention of infection by pathogens and parasites.
Anti-integrin αvβ6 antibody is frequently detected in UC patients, and its antibody titer has been reported to be correlated with disease activity※1.
The kit can be used in any laboratories which have absorbance microplate readers. Looking forward, this type of antibody testing could be used in diagnostic or therapeutic procedures, and it is expected that medical treatment can be readily provided without a specialist.
※1 Kuwada, T., et al. (2021). "Identification of an Anti-Integrin αvβ6 Autoantibody in Patients With Ulcerative Colitis." Gastroenterology 160(7): 2383-2394.e2321.
Product information → https://www.mblbio.com/bio/g/dtl/P/?pcd=5288
Purchase order → https://www.mblbio.com/e/inq/ivd.html
Head Quarter: SUMITOMO FUDOSAN SHIBADAIMON NICHOME BLDG. 2-11-8 Shibadaimon, Minato-ku, Tokyo 105-0012 Japan
Medical & Biological Laboratories (MBL) was established in 1969 as the first antibody manufacturer in Japan, and researches, develops, manufactures, and sells reagents of in vitro diagnostics (IVD) and reagents for basic research. It has now expanded its business not only to the immunological field but also to the field of genetic diagnosis. In the IVD business, we develop and sell IVD reagents for autoimmune diseases, cancer, infectious diseases, etc. Particularly in the field of autoantibody diagnosis, we are expanding our product lineup as a top manufacturer in Japan and contributing to medical care in this field where there are many intractable diseases. In the field of oncology, we are contributing to personalized medicine by developing companion diagnostics that predict the effects of drugs.
Contact details
-
Download vCard
- Sarah Martin-Tyrrell
-
JSR Life Sciences Company Inquiries
Senior Manager, PR and Content - sarah.martin-tyrrell@crownbio.com
Related topics
Related news
MBL Co-sponsors a Seminar on MEBRIGHT™ genitalium Plus DR Kit at the 98th JAID / the 72nd JSC Conference
MBL to co-sponsor a seminar on MEBRIGHT™ genitalium Plus DR Kit at the 98th JAID/72nd JSC Conference, focusing on STD treatment advancements. Learn more on June 27th, 2024.
MBL announces exclusive license agreement for Anti-Integrin αvβ6 Antibody with Immune-related Adverse Events colitis
MBL has announced that it has entered into an exclusive license agreement with Kyoto University and Kindai University regarding the detection of Anti-Integrin αvβ6 autoantibodies as an indicator of...
MEBGEN™ BRAF 2 Kit Receives MHLW Approval as a Companion Diagnostic for Use with Patients with Thyroid Cancer
With this approval, the MEBGEN™ BRAF 2 Kit is expected to contribute to the timely personalized treatment for patients with radically unresectable thyroid cancer based on the presence or absence of...
Notice of Approval for First-Class Marketing License for Medical Devices
With the acquisition of the First-Class Marketing License for Medical Devices, MBL will be able to market highly controlled medical devices, including genetic testing systems using NGS.
Launch of a New Chemiluminescence Immunoassay Analyzer “iStar500”
The iStar is a highly automated mono-test analyzer based on CLIA technology. The compact & integrated design, combined with highly sensitive CLIA technology, makes it an ideal choice for emergency ...